Our science is anything but basic
Breaking Barriers to Deliver pH Modulation Where it Matters
At Dyve Biosciences, we leverage our groundbreaking DMAX transdermal platform to deliver intuitive, targeted pH modulation to treat a range of immunologic and oncologic diseases. By directly targeting the acidic microenvironments that drive cancer cell growth and inflammation, we are taking decades of proven science from the petri dish to the patient.
Our Target: The Acidic Microenvironments That Drive Disease
In oncology, the acidic tumor microenvironment (TME) drives immune evasion and cancer progression.1 These acidosis-exposed cancer cells demonstrate high invasive ability and are especially resistant to apoptosis, or cell death, making them more resistant to therapy.2 Beyond oncology, glycolysis-derived acidic microenvironments have been shown to drive inflammation and progression in systemic sclerosis, rheumatoid arthritis, and other rheumatic diseases.3
Our lead products (DYV800 and DYV700) target acidic microenvironments to treat immunologic and oncologic diseases. Our patented approach to pH modulation increases the pH of an acidic microenvironment, effectively reversing the extracellular conditions that lead to disease progression. These systemically bioavailable products seek out locally acidic microenvironments where targeted neutralization of pH exerts a potent corrective effect via critical metabolic and hormonal functions leading to multi-fold better outcomes.3
Though many drug development companies have focused on reducing acid production in the TME or treating the implications of acidity such as poorer response to immunotherapy, successfully altering the pH of the TME in patients has remained a challenge largely because the acidity of the human gastrointestinal tract makes oral delivery untenable.4
Tumors thrive in an acidic microenvironment
Not All Drugs Are Suitable for Oral or Injectable Administration
In vitro research has demonstrated that many base chemotherapies and immunotherapies benefit from a more neutral TME, but delivery in patients has been a persistent challenge. While alkalinizing agents can be administered orally to treat conditions such as heartburn, clinical trials show that patients cannot tolerate the oral doses required to effectively treat cancer or tumor-related pain. Moreover, these agents are not appropriate for injectable administration due to the high potential for adverse events.

Pills. Needles. Dyve.
Understanding Dyve’s DMAX Platform
Dyve’s groundbreaking proprietary platform delivers pH-modulating agents and other molecules that are unsuitable for oral or injectable delivery and unable to cross epithelial barriers. Our novel emulsion technology transiently and reversibly fluidizes the skin’s lipid matrix and modulates tight junctions with topical application, allowing for nondestructive delivery and improved pharmacokinetics.
With the fast onset of action and systemic availability of an injectable and the convenience and optimal pharmacokinetics of a pill, DMAX enables us to work with a broad set of molecules such as pH modulators that are outside Lipinski’s Rules. Our deep understanding of emulsion chemistry allows us to push the boundaries of what can be delivered through the skin, establishing transdermal administration as a legitimate third route of drug delivery.
By using DMAX-enabled pH modulation, clinicians can take a more intuitive approach when treating patients with cutaneous tumors, as well as those with rheumatic joint and skin conditions in which acidity plays a significant role in disease progression and inflammation.

Demonstrated Efficacy
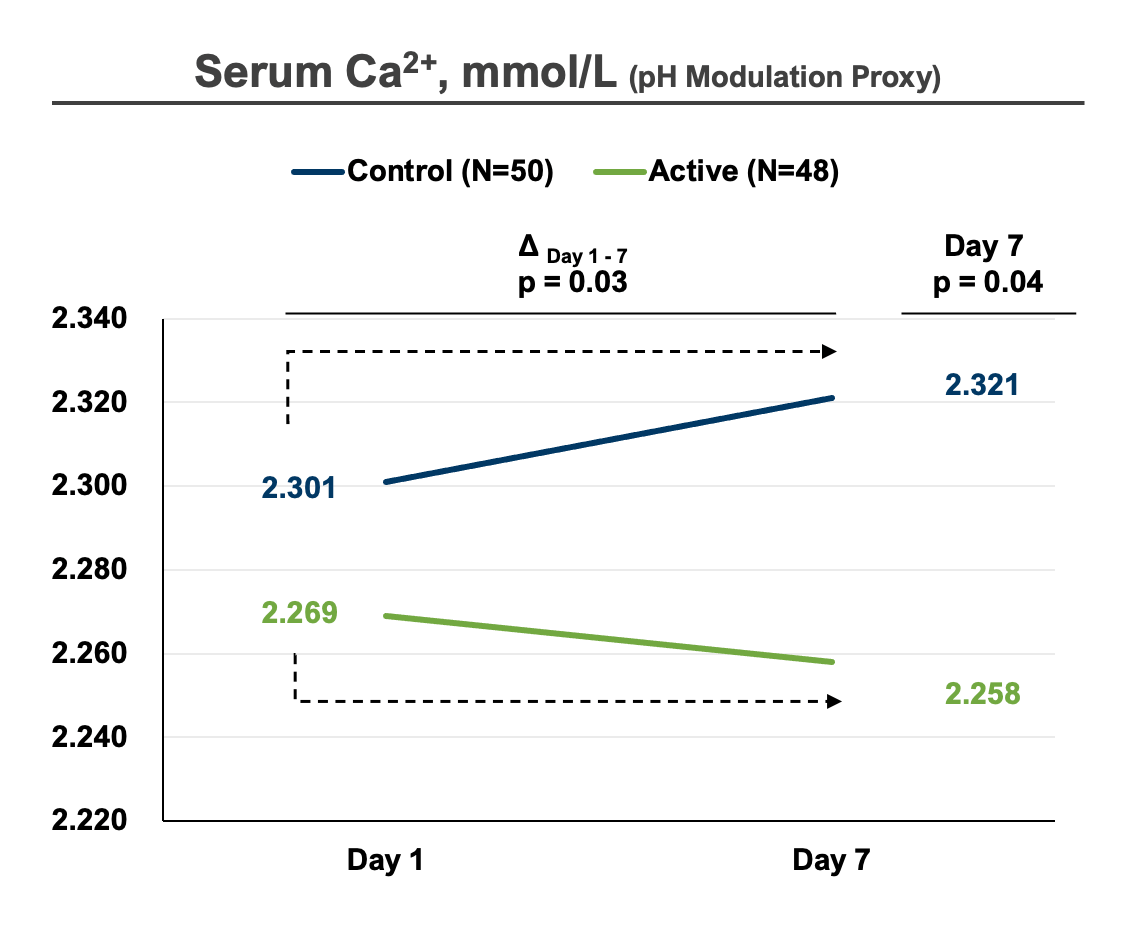
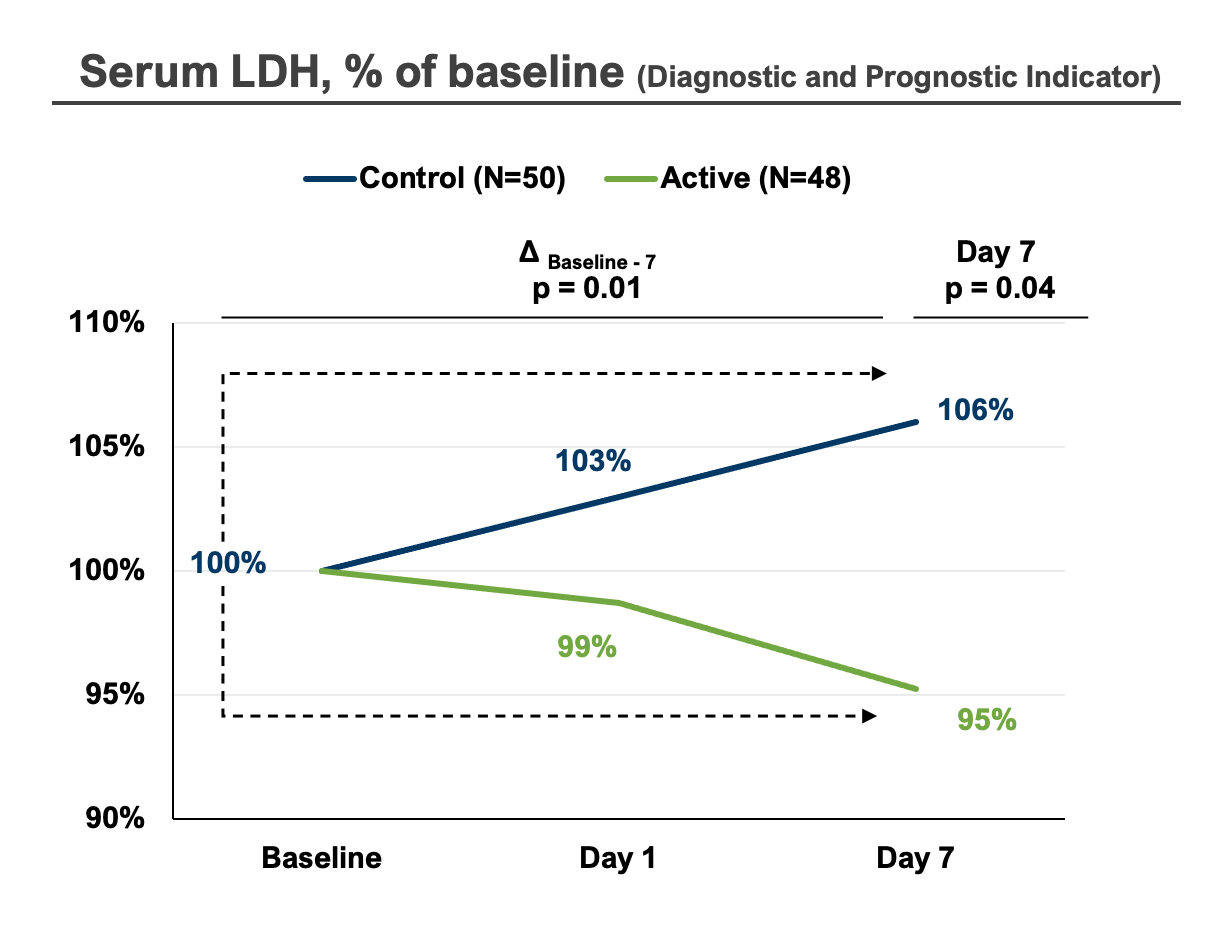
In 2022, Dyve completed a successful phase 2 study in which our proprietary DYV700 met its clinically relevant endpoint of reducing pain caused by the acidic microenvironment of acute gouty arthritis. Additionally, our biomarker data serves as proof of concept for the effectiveness of transdermal pH modulation of the acidic disease microenvironment.
Our Pipeline: Expanding the Treatment Horizon for a Range of Diseases
At Dyve, we leverage the skin for drug delivery to unlock the transformative potential of pH modulation and set a new standard of care across oncology and immunology. Dyve’s lead product, DYV800, delivers pH modulation to solid tumors to raise the pH of the acidic TME, potentially inhibiting tumor proliferation and boosting the efficacy of other therapies such as immune checkpoint inhibitors.5
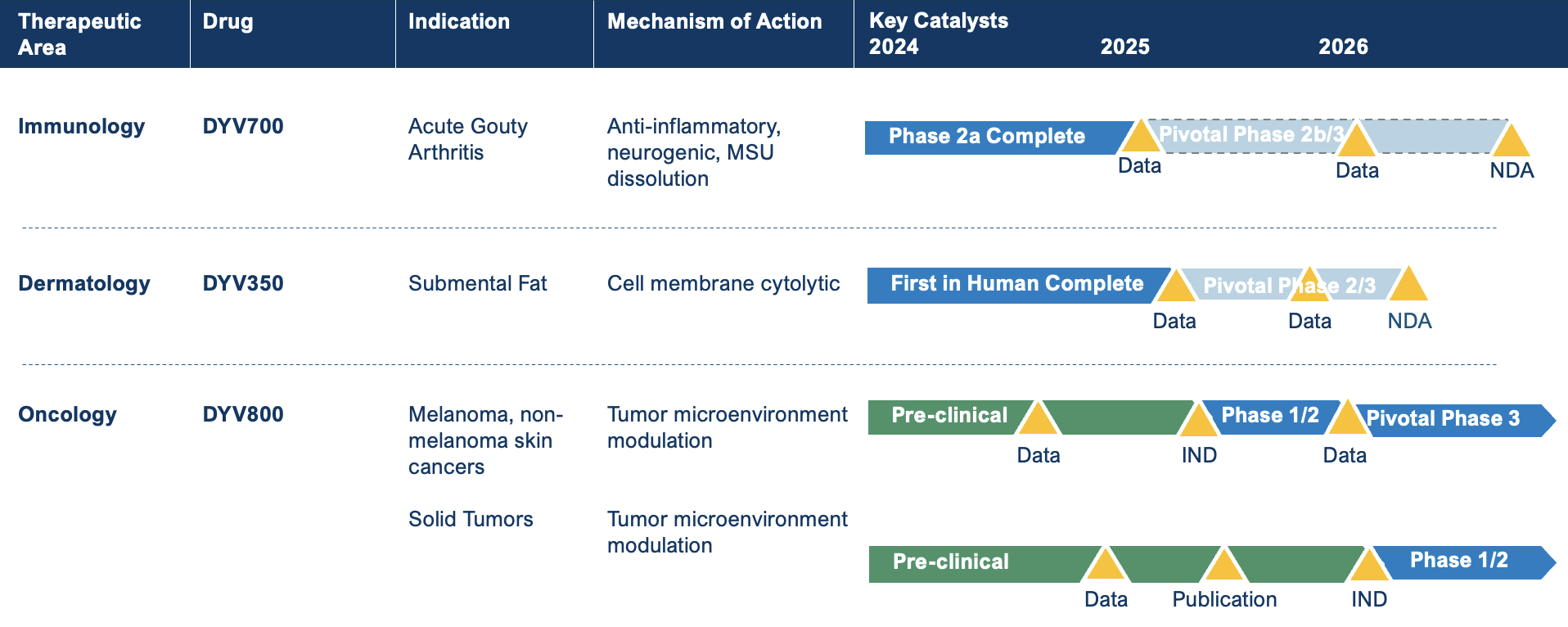
In addition to pH modulation, Dyve’s platform science can be applied to a wide array of approved therapies to provide improved therapeutic benefits to patients while avoiding first-pass metabolism and potentially minimizing systemic adverse events. Dyve’s proprietary science enables systemic drug delivery to treat diseases that affect the whole body, as well as targeted treatment for diseases in or near the skin’s surface.
If interested in partnership or collaboration opportunities please reach out using the Contact form below.

Our people
Dyve is led by a team of scientists, clinicians, and operators with decades of deep scientific, clinical, and pharmaceutical industry experience.

Board of Directors
Contact us
At Dyve, we are passionate about setting a new standard of care by creating therapies that are patient friendly and clinically intuitive for an array of indications. Learn more about how you can join our team and push the limits of what’s possible.
References:
Lee SH, Griffiths JR. How and why are cancers acidic? Carbonic anhydrase IX and the homeostatic control of tumour extracellular pH. Cancers (Basel). 2020;12(6):1616. doi:10.3390/cancers12061616
Andreucci E, Peppicelli S, Ruzzolini J, et al. The acidic tumor microenvironment drives a stem-like phenotype in melanoma cells. J Mol Med (Berl). 2020;98(10):1431-1446. doi:10.1007/s00109-020-01959-y
Andreucci E, Margheri F, Peppicelli S, et al. Glycolysis-derived acidic microenvironment as a driver of endothelial dysfunction in systemic sclerosis. Rheumatology (Oxford). 2021;60(10):4508-4519. doi:10.1093/rheumatology/keab022
Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016;76(6):1381-1390. doi:10.1158/0008-5472.CAN-15-1743. Erratum in: Cancer Res. 2017;77(9):2552. doi:10.1158/0008-5472.CAN-17-0559
- Liu J, Chen Q, Feng L, Liu Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today. 2018;21:55-73. https://doi.org/10.1016/j.nantod.2018.06.008.